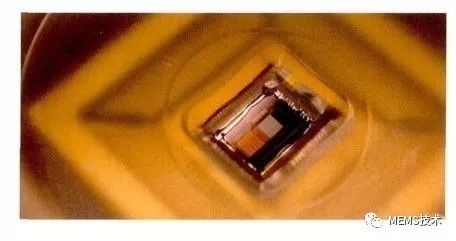
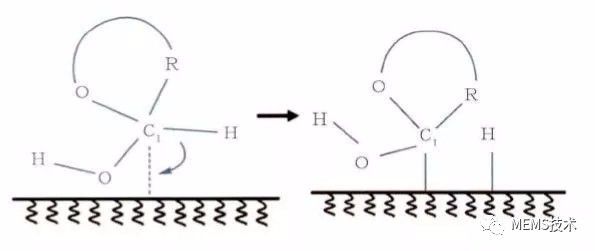
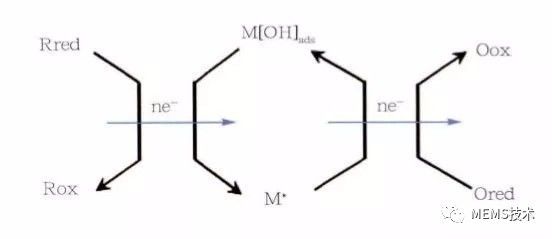
Biosensor technology has high specificity and sensitivity, and is widely used in online analysis and detection of complex systems. It has good development prospects in clinical diagnosis, analytical chemistry, food testing, medical analysis, and chemical industry. Biosensor technology is a branch of analytical biology that permeates many disciplines such as analytical chemistry, biology, life sciences, and physics. For biosensors, there are two main parts, namely the identification system and the signal conversion system. The specific substance can react specifically with the sensor through the identification system, which is the key to the identification of the sensor, highlighting the specificity of the detected substance. As the detection system detection substance, it may be a protein, an enzyme, an antigen antibody, a DNA, a nucleic acid, a biofilm, a cell, a tissue, a microorganism, etc., and the biosensor may be classified into an enzyme sensor, an immunosensor, a cell sensor, etc. according to the type of the identification material. . Another part of the signal conversion system is to convert the specific reaction between the specific substance and the recognition system into information that we can recognize (such as light, heat, electricity information) to amplify and output, and according to the way of signal conversion, the sensor can be divided into photobio Sensors, electrochemical biosensors, etc. Because the electrical signal has the characteristics of fast response, easy conversion and simple data analysis, electrochemical biosensor has become the earliest development, the research content and the most abundant, the most widely used sensor. The electrochemical biosensor mainly uses an electrode as an information conversion material to convert a substance-specific reaction process into an electrical signal, and indirectly expresses the concentration of the reactant by the magnitude of the electrical signal. Among them, the development of enzyme electrodes is the most representative in the field of biosensors. Enzyme electrode is the most widely studied biosensor, mainly due to its high sensitivity, good specificity, simple instrument and fast response. The enzyme electrode biosensor refers to a biological enzyme as a recognition unit, and the biological enzyme is immobilized on the surface of the modified electrode. When a specific substance corresponding to a biological enzyme is oxidized in the test substrate, the reaction process generates electron exchange on the surface of the electrode, and the chemical reaction occurs by detecting the change of the current to represent the read substance. The concentration changes. However, biological enzymes usually have redox active centers composed of one or several metal ions, and most of the active centers are deeply buried in the protein peptide chain, making it difficult for the enzyme active center to directly exchange electrons with the electrode surface. In order to solve the problem of charge transfer between the active center of the enzyme and the electrode, the bioenzyme electrochemical sensor has three main stages of development. The first stage of the enzyme electrode uses oxygen as the electron acceptor, and the glucose oxidase sensor as an example. The reaction process is as follows (1), (2). GOx (FAD) oxidized glucose enzyme oxidizes glucose to glucose lactone, while the reduced enzyme GOx (FADH2) reduces oxygen in solution to hydrogen peroxide by measuring the change in concentration of oxygen or hydrogen peroxide during the reaction. The glucose concentration was measured indirectly. However, the sensor at this stage is highly susceptible to oxygen in the environment and has poor anti-interference ability. GOx (FAD) + glucose→GOx(FADH2)+ glucolactone (1) GOx(FADH2) + O2→GOx(FAD) + H2O2 (2) The sensor of the second stage is to increase the mediator layer for electron transfer between the bio-enzyme and the electrode, and replace the oxygen as an electron, which overcomes the problem of interference. The mediator material capable of rapidly performing the redox reaction is used as an intermediate for electron transfer between the active center of the enzyme and the surface of the electrode, and the reaction process is as follows (3), (4), and (5). The oxidation state enzyme oxidizes the substrate to a reduced state enzyme, and at the same time, the process of reducing and oxidizing the mediator substance transfers the reaction charge to the surface of the electrode, and the concentration of the reaction substrate indicates the concentration of the reaction substrate. However, the mediator material is easily diffused, which puts higher demands on the fixation of the mediator material. GOx (FAD) + glucose→GOx(FADH2) + glucolactone (3) GOx(FADH2) + 2Medox + 2e-→GOx (FAD) + 2Medred + 2H+ (4) 2Medred→2Medox + 2e- (5) The third stage enzyme electrode sensor does not require oxygen or a mediator as an electron acceptor, but chemically opens the enzyme protein peptide chain to expose the enzyme active center or special treatment of the electrode surface to immobilize the biological enzyme on the electrode surface. The charge exchange is directly carried out with the electrode while catalyzing the oxidation reaction, and the reaction process is as follows (6), (7). However, the efficiency of electron transport by the nature of the enzyme itself is still limited. GOx (FAD) + glucose→GOx(FADH2)+glucolactone (6) GOx(FADH2 ) + 2e-→GOx(FAD)+ 2H+ (7) In the enzyme electrode sensor, the activity of the enzyme is a key factor determining the stability and sensitivity of the sensor, but it is easily denatured and inactivated during the enzyme immobilization process, and the activity of the enzyme is also susceptible to the surrounding environment such as humidity, temperature, and chemical factors. And the enzyme may leak during the fixation process, and some biological enzymes cost more. Therefore, a method for catalyzing the oxidation of a test object by using a metal, a metal oxide, an alloy or the like having a plurality of oxidation valence states as a catalytic material instead of immobilizing the biological enzyme on the surface of the electrode is proposed. There are two oxidation mechanisms that are generally accepted for the theory that materials catalyze oxidation on the electrode surface. Take glucose as an example of oxidation at the electrode. The first is the adsorption theory of adjacent sites. When the glucose adsorbed on the surface of the electrode is oxidized, the CH bond on the hemiacetal carbon in the glucose molecule is broken, and the hydrogen atom and the hemiacetal carbon simultaneously form a chemical bond on the surface of the electrode, such as Figure 1 shows. Figure 1 Adsorption of adjacent sites The second is the theory of intermediate oxidation. Metal atoms form a metal ion membrane when adsorbed by glucose molecules. Metal ions oxidize the adsorbed glucose molecules to glucose lactone acid, and the ion membrane is reduced to metal atoms on the surface of the electrode to realize charge exchange. as shown in picture 2. Figure 2 shows the oxidation of the intermediate At present, there are many studies on enzyme-free sensors, and glucose-free enzyme sensors are the most widely studied. For example, there are enzyme-free sensors using noble metals Pt, Au, and Pd as glucose catalytic materials, and transition metals such as Ni, Cu, and oxides thereof are modified to prepare sensor electrodes, and various metals or oxides are made into hybrid electrodes. . Although the enzyme-free sensor is not affected by the enzyme activity, there are some problems, such as the high cost of precious metals Pt and Au. Although it has good catalytic activity for glucose, Cl- in the solution is easily adsorbed on the electrode surface; Pd The nanoparticles are easily polymerized; the transition metals Ni, Cu, and their oxides have higher sensitivity, but have a narrow linear range for detecting glucose. Enzyme-free sensors are highly susceptible to the chemical environment and have high requirements for the detection environment, so they are generally tested in buffer solutions.
Power 12W ,output voltage 3-12V, output current Max 1A, 6 dc tips. We can meet your specific requirement of the products, like label design. The material of this product is PC+ABS. All condition of our product is 100% brand new.
Our products built with input/output overvoltage protection, input/output overcurrent protection, over temperature protection, over power protection and short circuit protection. You can send more details of this product, so that we can offer best service to you!
12W Wall Adapter, 12W Wall Power Supply,12W Power Cord In Wall, 12W Wall Power Adapter Shenzhen Waweis Technology Co., Ltd. , https://www.waweis.com