1. The potential of copper and aluminum is different. The contact between copper and aluminum will accelerate the oxidation of the aluminum wire due to the reaction of the primary battery. For a long time, the copper-aluminum joint will be in poor contact. To be connected together, you must use copper and aluminum over-clamps or over-line tubes. 2. This is a chemical problem. The chemical properties of metals are relatively lively and inactive. For example, gold is never rusted. This means that gold is chemically inert, iron is easy to rust, and iron is more lively than gold. When the metals are put together, the oxidation of the active metal is accelerated. Compared with copper, the aluminum is more active. The connection of the two cables accelerates the oxidation of the aluminum (that is, rust) and affects the use. 3. When the copper and aluminum conductors are directly connected, the contact surfaces of the two metals are easily formed into an electrolyte under the action of moisture, carbon dioxide and other impurities in the air, thereby forming a primary battery with aluminum as the negative electrode and copper as the positive electrode. , causing galvanic corrosion of aluminum, resulting in increased contact resistance at the junction of copper and aluminum. (copper aluminum wire connector) In addition, since the elastic modulus and thermal expansion coefficient of copper and aluminum differ greatly, after a plurality of thermal cycles (energization and power-off) during operation, a large gap is generated at the contact point to affect the contact, and the contact is also increased. Large contact resistance. An increase in contact resistance causes an increase in temperature during operation. Corrosion oxidation is intensified at high temperatures, causing a vicious cycle, which further deteriorates the quality of the connection, and finally causes the contact point temperature to be too high and may even cause smoke, burnout and the like. Question: Why use copper and aluminum transition clamps when connecting copper wire and aluminum wire? 1. The grayish white material formed at the junction of the copper wire and the aluminum wire is slightly longer, and the contact resistance at the joint will increase and heat, which may cause a building fire when the circuit is broken. So use a special copper-aluminum transition clamp. Avoid unnecessary trouble! Copper and aluminum wire connection device (commonly known as "copper aluminum nose") 2. If the copper wire and the aluminum wire are directly hinged together, the joint is easily oxidized, resulting in an increase in electrical resistance and thus more likely to burn out. The real reason for this damage is that the aluminum element is more active than the copper element, and a much micro-potential (i.e., micro-battery) is generated on the copper-aluminum bonding surface, thereby causing micro-electro-erosion, and the contact resistance becomes large over time. In ordinary homework, it is more reliable to connect the copper to the tin. 3. In the power system, copper and aluminum are directly connected, and galvanic corrosion occurs when current flows. Therefore, copper-aluminum transition should be used, or a tin sheet should be placed in the middle of the contact surface, and a layer of conductive paste should be applied. When the ordinary household is connected, the copper wire can be connected to the aluminum wire and connected to the aluminum wire to avoid galvanic corrosion. Fiber Optic Pigtail,Pigtail Patch Cord,Fiber Pigtail,Pigtail Fiber Optik Huizhou Fibercan Industrial Co.Ltd , https://www.fibercan-network.com

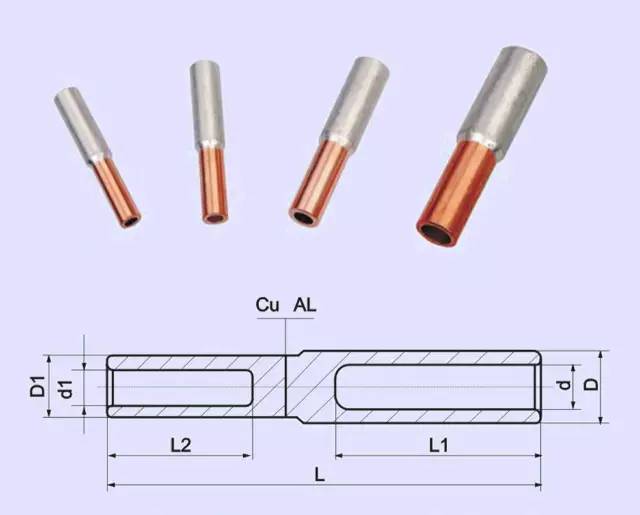



Question: Why can't the aluminum wire be connected to the copper wire?